Innovative air cleaning leader conducts a series of tests to show efficacy of DFS technology.
Pulaski, NY, August 11, 2020– In response to the COVID-19 Pandemic, HealthWay Family of Brands has conducted a series of tests to show the efficacy of DFS technology on the capture and permanent removal of airborne viruses and bacteria. World-renowned Infection Control expert and an advisor to the U.S. EPA (Environmental Protection Agency) and the World Health Organization, Dr. Syed Sattar, conducted his studies in a chamber that was built to follow U.S. EPA guidelines.
Important Note
During an outbreak of a new virus like COVID-19, no products exist whether it be a surface disinfectant or an air treatment device that can make claims to kill or capture the virus. This is due to the simple fact that the virus was not available to test, and it can take more than 1 year to get a viral claim approved by a regulatory agency. For this reason, the U.S. enacted a ‘hierarchy-based’ policy. Should a company’s product be found to be effective against harder to kill or capture viruses, it is likely to kill or capture a virus like COVID-19. A product that is likely to provide the greatest protection to you from COVID-19 will have claims against at least one non-enveloped virus such as Norovirus, Feline Calicivirus, Poliovirus, Rhinovirus or Reovirus.
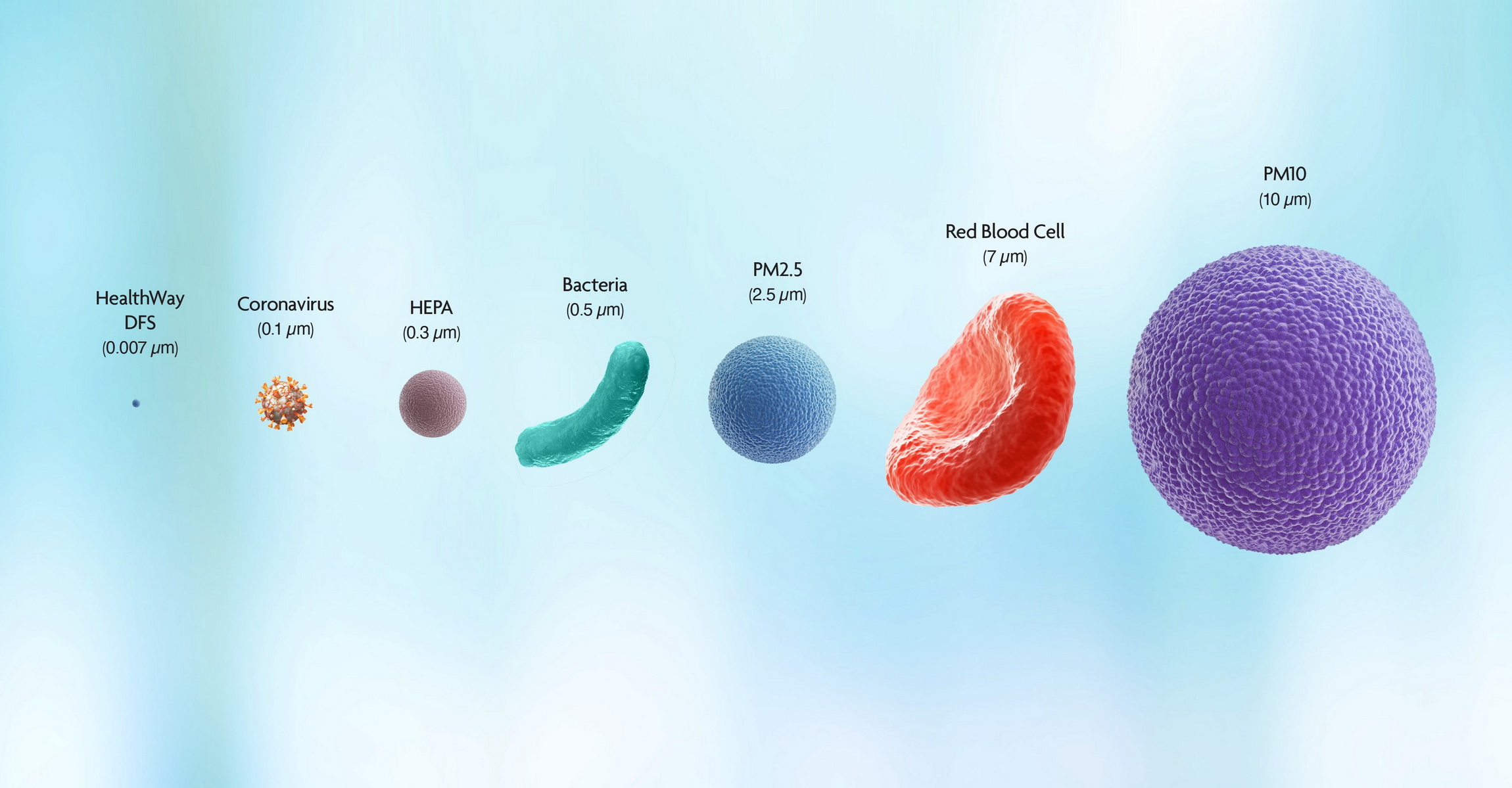
Test Conclusions
DFS technology was proven to remove from the air a proxy virus for SARS-CoV-2 (RNA virus MS2)1: The results of this data show 99.987% elimination in 10 minutes of the surrogate which is .03 micron in size. The test also showed that there was 100% elimination in 45 minutes and again 100% at 24 hours indicating no reintroduction of the proxy virus. The bacteriophage MS-2 is a small (about 30 nanometers or .03 micron), non- enveloped virus, which is often used as a surrogate for small, non-enveloped human pathogenic viruses such as noroviruses.Testing with Bacteriophage Phi6 as the Challenge, independent testing indicated a 99.99% reduction in 10 minutes. Bacteriophage Phi6 is a larger (approximately 100 nanometers or .1 micron), enveloped virus, which is often used to represent large, enveloped human pathogenic viruses such as coronaviruses and influenza viruses and is most similar in size and composition to SARS-CoV-2. both tests indicate substantial virus elimination within 10 minutes across a range of sizes and composition of both enveloped and non-enveloped viruses.
About HealthWay Family of Brands
For over 40 years, HealthWay Family of Brands has been reimagining the world as a better, safer place with innovative air purification technologies. Our solutions are crafted in the USA and trusted everywhere air quality matters. Indoor air quality problems come in all shapes and sizes – we’re proud that our industry-leading patented DFS (Disinfecting Filtration System) technology is being used in cleanroom applications, hospital surgical suites, commercial office buildings, hotels, schools, universities and many mission-critical environments around the globe. To learn more, contact Christian Cobb at ccobb@healthway.com.


Comments are closed